
Mastrole (anastrozole) tablet for oral use
DESCRIPTION
MASTROLE (anastrozole) tablets for oral administration contain 1 mg of anastrozole, a non-steroidal aromatase inhibitor. It is chemically described as 1,3-Benzenediacetonitrile, a, a, a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl). Its molecular formula is C17H19N5 and its structural formula is:
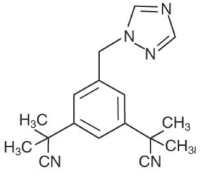
Anastrozole is an off-white powder with a molecular weight of 293.4. Anastrozole has moderate aqueous solubility (0.5 mg/mL at 25°C); solubility is independent of pH in the physiological range. Anastrozole is freely soluble in methanol, acetone, ethanol, and tetrahydrofuran, and very soluble in acetonitrile
CLINICAL PHARMACOLOGY
MECHANISM OF ACTION
- The growth of many cancers of the breast is stimulated or maintained by estrogens.
- In postmenopausal women, estrogens are mainly derived from the action of the aromatase enzyme, which converts adrenal androgens (primarily androstenedione and testosterone) to estrone and estradiol. The suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
- Anastrozole is a selective non-steroidal aromatase inhibitor. It significantly lowers serum estradiol concentrations and has no detectable effect on formation of adrenal corticosteroids or aldosterone
INDICATIONS AND USAGE
Mastrole is an aromatase inhibitor indicated for:
- Adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer.
- First-line treatment of postmenopausal women with hormone receptor-positive or hormone receptor unknown locally advanced or metastatic breast cancer.
- Treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. Patients with ER-negative disease and patients who did not respond to previous tamoxifen therapy rarely responded to Mastrole.
DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
- The dose of Mastrole is one 1 mg tablet taken once a day. For patients with advanced breast cancer, Mastrole should be continued until tumor progression. Mastrole can be taken with or without food.
- For adjuvant treatment of early breast cancer in postmenopausal women, the optimal duration of therapy is unknown. In the ATAC trial, Anastrozole was administered for five years.
- No dosage adjustment is necessary for patients with renal impairment or for elderly patients.
2.2 Patients with Hepatic Impairment
- No changes in dose are recommended for patients with mild-to-moderate hepatic impairment. Anastrozole has not been studied in patients with severe hepatic impairment.
HOW SUPPLIED/STORAGE AND HANDLING
These tablets are supplied in Box of 10*1*10 strip.
Storage
Store at controlled room temperature, 20-25°C (68-77°F)
MASTROLE is a registered trademark of the Masvik Pharmaceuticals Private Limited. All rights reserved.